
What is a calorimeter? Name the material of which it is made of. Give two reasons for using the material stated by you.
Answer
534.9k+ views
Hint: Calorimeter is a device which is used for the science of calorimetry. The calorimetry is the branch of science that deals with determining the change in the state variables of a body for the purpose of deriving the value of heat transfer during a thermodynamic process.
Complete step-by-step answer:
During a physical or a chemical reaction, it is natural that there is heat transfer towards and from the reaction system. This heat energy in the form of a quantity called enthalpy is measured using the device called calorimeter.
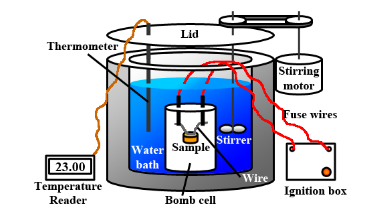
To calculate the enthalpy, the reactants are put into the calorimeter separately and the reaction is ignited. The initial and final temperatures are recorded and the formula is used to calculate the enthalpy change –
$\Delta H = C\Delta t$
where C – specific heat capacity of the materials used and $\Delta t$ is the change in the temperature.
The material used for calorimeter construction is copper.
The main reason for using copper as the material for construction of the calorimeter is –
High Thermal conductivity:
Since the heat has to be conducted evenly, uniformly, and quickly to the materials, the material used, should have a higher value of thermal conductivity. Copper has one of the best thermal conductivities among the metals.
Low specific heat capacity:
Copper has low thermal specific heat capacity because it should not take in the heat to increase its own temperature, thereby adding to the heat produced and producing error in the calculation of the enthalpy change.
Note: In this answer, only the generic specific heat capacity is mentioned as C. However, there are different values of specific heat when the system is at constant volume and constant pressure.
${C_v}\& {C_p}$ are the specific heats at constant volume and pressure respectively. In this case of calorimeter, the specific heat taken is of ${C_v}$ since the calorimeter when closed, becomes a constant volume enclosure for the reaction to happen at constant volume condition.
The difference between ${C_p}\& {C_v}$ is equal to the universal gas constant R.
${C_p} - {C_v} = R$
Complete step-by-step answer:
During a physical or a chemical reaction, it is natural that there is heat transfer towards and from the reaction system. This heat energy in the form of a quantity called enthalpy is measured using the device called calorimeter.

To calculate the enthalpy, the reactants are put into the calorimeter separately and the reaction is ignited. The initial and final temperatures are recorded and the formula is used to calculate the enthalpy change –
$\Delta H = C\Delta t$
where C – specific heat capacity of the materials used and $\Delta t$ is the change in the temperature.
The material used for calorimeter construction is copper.
The main reason for using copper as the material for construction of the calorimeter is –
High Thermal conductivity:
Since the heat has to be conducted evenly, uniformly, and quickly to the materials, the material used, should have a higher value of thermal conductivity. Copper has one of the best thermal conductivities among the metals.
Low specific heat capacity:
Copper has low thermal specific heat capacity because it should not take in the heat to increase its own temperature, thereby adding to the heat produced and producing error in the calculation of the enthalpy change.
Note: In this answer, only the generic specific heat capacity is mentioned as C. However, there are different values of specific heat when the system is at constant volume and constant pressure.
${C_v}\& {C_p}$ are the specific heats at constant volume and pressure respectively. In this case of calorimeter, the specific heat taken is of ${C_v}$ since the calorimeter when closed, becomes a constant volume enclosure for the reaction to happen at constant volume condition.
The difference between ${C_p}\& {C_v}$ is equal to the universal gas constant R.
${C_p} - {C_v} = R$
Recently Updated Pages
Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Trending doubts
Select the word that is correctly spelled a Twelveth class 10 english CBSE

Choose the feminine form of the given noun Fox AFoxess class 10 english CBSE

Write examples of herbivores carnivores and omnivo class 10 biology CBSE

Who was the first Indian ruler to enter into the Subsidiary class 10 social science CBSE

List out three methods of soil conservation

Why is it 530 pm in india when it is 1200 afternoon class 10 social science CBSE




