
The potential energy of an electron in the hydrogen atom is $ - 6.8eV.$Indicate in which excited state, the electron in present?
A. First
B. Second
C. Third
D. Fourth
Answer
468.3k+ views
Hint:Before answering the question let us first understand about the potential energy of electrons. The motion of electrons gives atoms their kinetic and potential energy. When electrons are excited, they travel away from the atom to a higher energy orbital. The potential energy is released in the form of kinetic energy when the electron returns to a low energy state.
Formula used:
The potential energy of an electron is calculated using the formula
\[{E_n} = - 13.6\dfrac{{{Z^2}}}{n}\],
where $Z$ is the atomic number and $n$ is the shell.
Complete step by step answer:
The cumulative energy of the electrons in an excited-state atom can be reduced by passing one or more electrons to different orbitals. By transferring an electron from a 2P orbital to a 2S orbital, the total energy of the electrons in this carbon atom can be reduced. Let us see the energy state of the hydrogen atom.
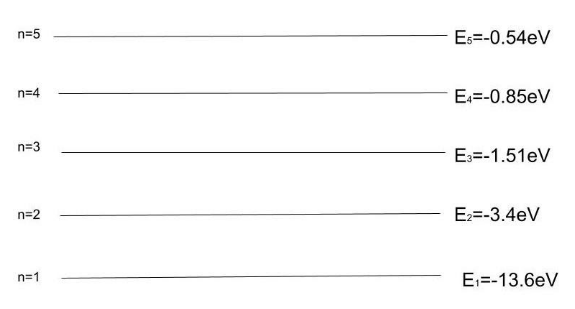
We know that energy in the ${E_n}$ is equal to the half of the potential energy of the electron.
${E_n} = \dfrac{1}{2}P.E$
$\Rightarrow {E_n} = \dfrac{{ - 6.8eV}}{2} \\
\Rightarrow {E_n} = - 3.4eV$
So the second energy state of the hydrogen atom has energy $ - 3.4eV$.
$\therefore n = 2 \\$
Hence, the correct answer is option B.
Note: Let us get some more information about the energy state of hydrogen. Bohr calculated the energy of an electron in the nth energy level of hydrogen by holding electrons in circular, quantized orbits around the positively charged nucleus.
Formula used:
The potential energy of an electron is calculated using the formula
\[{E_n} = - 13.6\dfrac{{{Z^2}}}{n}\],
where $Z$ is the atomic number and $n$ is the shell.
Complete step by step answer:
The cumulative energy of the electrons in an excited-state atom can be reduced by passing one or more electrons to different orbitals. By transferring an electron from a 2P orbital to a 2S orbital, the total energy of the electrons in this carbon atom can be reduced. Let us see the energy state of the hydrogen atom.

We know that energy in the ${E_n}$ is equal to the half of the potential energy of the electron.
${E_n} = \dfrac{1}{2}P.E$
$\Rightarrow {E_n} = \dfrac{{ - 6.8eV}}{2} \\
\Rightarrow {E_n} = - 3.4eV$
So the second energy state of the hydrogen atom has energy $ - 3.4eV$.
$\therefore n = 2 \\$
Hence, the correct answer is option B.
Note: Let us get some more information about the energy state of hydrogen. Bohr calculated the energy of an electron in the nth energy level of hydrogen by holding electrons in circular, quantized orbits around the positively charged nucleus.
Recently Updated Pages
Master Class 12 Business Studies: Engaging Questions & Answers for Success

Master Class 12 Biology: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Class 12 Question and Answer - Your Ultimate Solutions Guide

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What is transplantation in agriculture class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

Why is the cell called the structural and functional class 12 biology CBSE

Who discovered the cell and how class 12 biology CBSE

What is the Full Form of PVC, PET, HDPE, LDPE, PP and PS ?




